What Is The Makeup Of The Atmosphere
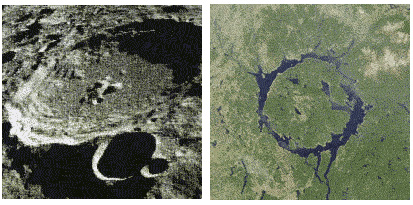
The fact that the moon'south surface is covered with meteorite impact craters is obvious to us today. Though the moon is not far from us, impact craters are few and far betwixt on Earth. As it turns out, World has received but as many incoming meteorites as the moon, merely the presence of the temper has determined the fate of many of them. Small meteorites fire up in the atmosphere before ever reaching Earth. Those that do hit the surface and create an touch on crater are lost to us in a different way – the craters are quickly eroded by weather generated in the atmosphere, and the show is washed away. The moon, on the other paw, has no temper, and thus every falling star aimed at the moon hits information technology, and the craters have remained essentially unchanged for iv billion years (Effigy 1).
Composition of Earth's atmosphere
The early on Greeks considered "air" to be ane of four simple substances; along with earth, burn down, and water, air was viewed as a key component of the universe. By the early 1800s, however, scientists such equally John Dalton recognized that the atmosphere was in fact composed of several chemically distinct gases, which he was able to separate and make up one's mind the relative amounts of within the lower atmosphere. He was easily able to discern the major components of the atmosphere: nitrogen, oxygen, and a small corporeality of something incombustible, later shown to exist argon.
The evolution of the spectrometer in the 1920s allowed scientists to find gases that existed in much smaller concentrations in the temper, such as ozone and carbon dioxide. The concentrations of these gases, while pocket-sized, varied widely from place to place. In fact, atmospheric gases are ofttimes divided up into the major, abiding components and the highly variable components, as shown in Tabular array 1 and Tabular array ii.
| Nitrogen (Northwardii) | 78.08% |
| Oxygen (O2) | 20.95% |
| Argon (Ar) | 0.93% |
| Neon, Helium, Krypton | 0.0001% |
Tabular array one: Abiding Components. Proportions remain the same over time and location.
| Carbon dioxide (COtwo) | 0.038% |
| Water vapor (HtwoO) | 0-4% |
| Marsh gas (CHfour) | trace |
| Sulfur dioxide (Thenii) | trace |
| Ozone (O3) | trace |
| Nitrogen oxides (NO, NO2, Northwardtwo0) | trace |
Table 2: Variable Components. Amounts vary over fourth dimension and location.
Although both nitrogen and oxygen are essential to human being life on the planet, they have footling effect on weather and other atmospheric processes. The variable components, which make up far less than 1 percent of the temper, have a much greater influence on both short-term weather and long-term climate. For example, variations in water vapor in the atmosphere are familiar to the states as relative humidity. Water vapor, CO2, CH4, N2O, and So2 all have an important holding: They absorb heat emitted by Earth and thus warm the temper, creating what we call the "greenhouse effect." Without these and then-called greenhouse gases, the Globe's surface would exist about 30 degrees Celsius cooler – as well cold for life to exist as we know information technology. Though the greenhouse effect is sometimes portrayed as a bad matter, trace amounts of gases like COii warm our planet's atmosphere enough to sustain life. Global warming, on the other paw, is a dissever procedure that tin be caused by increased amounts of greenhouse gases in the atmosphere.
In addition to gases, the atmosphere also contains particulate matter such as grit, volcanic ash, rain, and snowfall. These are, of class, highly variable and are by and large less persistent than gas concentrations, simply they can sometimes remain in the atmosphere for relatively long periods of fourth dimension. Volcanic ash from the 1991 eruption of Mt. Pinatubo in the Philippines, for example, darkened skies around the globe for over a year.
Though the major components of the temper vary little today, they have changed dramatically over Globe's history, about 4.6 billion years. The early on atmosphere was inappreciably the life-sustaining blanket of air that it is today; nigh geologists believe that the main constituents then were nitrogen gas and carbon dioxide, but no costless oxygen. In fact, there is no evidence for free oxygen in the atmosphere until about two billion years ago, when photosynthesizing bacteria evolved and began taking in atmospheric carbon dioxide and releasing oxygen. The corporeality of oxygen in the atmosphere has risen steadily from 0 percent ii billion years ago to nearly 21 percent today.
Comprehension Checkpoint
Nitrogen and oxygen, which make upwardly more than than 99% of World's temper, have a bigger influence on climate than other components of the atmosphere.
Measuring the atmosphere
Nosotros now have continuous satellite monitoring of the temper and Doppler radar to tell united states whether or not we will experience pelting anytime before long; however, atmospheric measurements used to exist few and far between. Today, measurements such as temperature and pressure not only assist us predict the weather, simply as well help us look at long-term changes in global climate (come across our Temperature module). The offset atmospheric scientists were less concerned with conditions prediction, however, and more interested in the composition and structure of the atmosphere.
The 2 most important instruments for taking measurements in Earth'south atmosphere were developed hundreds of years ago: Galileo is credited with inventing the thermometer in 1593, and Evangelista Torricelli invented the barometer in 1643. With these two instruments, temperature and pressure could be recorded at any time and at any identify. Of course, the earliest pressure and temperature measurements were taken at World's surface. It was a hundred years before the thermometer and barometer went aloft.
While many people are familiar with Ben Franklin'southward kite and fundamental experiment that tested lightning for the presence of electricity, few realize that kites were the master vehicle for obtaining atmospheric measurements above Earth'due south surface. Throughout the 18thursday and nineteenth centuries, kite-mounted instruments collected pressure level, temperature, and humidity readings; unfortunately, scientists could simply reach up to an distance of about 3 km with this technique.

Unmanned balloons were able to take measurements at higher altitudes than kites, merely considering they were simply released with no passengers and no strings attached, they had to exist retrieved in club to obtain the data that had been collected. This changed with the development of the radiosonde, an unmanned balloon capable of achieving high altitudes, in the early on 1930s. The radiosonde included a radio transmitter among its many instruments, allowing information to be transmitted every bit it was being collected and so that the balloons no longer needed to exist retrieved. A radiosonde network was adult in the United States in 1937, and continues to this day under the auspices of the National Weather Service.
Comprehension Checkpoint
What was an advantage of the radiosonde over earlier data collection instruments?
Temperature in the atmosphere
Through examination of measurements collected by radiosonde and aircraft (and later on past rockets), scientists became aware that the atmosphere is not uniform. Many people had long recognized that temperature decreased with distance – if y'all've e'er hiked upwards a alpine mountain, you might learn to bring a jacket to wear at the top even when it is warm at the base – only it wasn't until the early on 1900s that radiosondes revealed a layer, about 18 km in a higher place the surface, where temperature abruptly inverse and began to increase with altitude. The discovery of this reversal led to division of the atmosphere into layers based on their thermal properties.
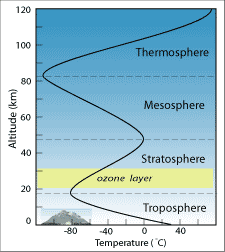
The lowermost 12 to 18 km of the atmosphere, called the troposphere, is where all weather occurs – clouds class and precipitation falls, wind blows, humidity varies from identify to place, and the atmosphere interacts with the surface below. Inside the troposphere, temperature decreases with altitude at a rate of near 6.v° C per kilometer. At 8,856 m high, Mt. Everest still reaches less than halfway through the troposphere. Assuming a sea level temperature of 26° C (lxxx° F), that ways the temperature on the summit of Everest would exist around -31° C (-24° F)! In fact, temperature at Everest's height averages -36° C, whereas temperatures in New Delhi (in nearby Republic of india), at an elevation of 233 m, average nearly 28° C (82.iv° F).
At the uppermost boundary of the troposphere, air temperature reaches about -100° C and then begins to increase with distance. This layer of increasing temperature is chosen the stratosphere. The cause of the temperature reversal is a layer of concentrated ozone. Ozone'due south ability to blot incoming ultraviolet (UV) radiations from the sun had been recognized in 1881, but the existence of the ozone layer at an altitude of 20 to 50 km was not postulated until the 1920s. By arresting UV rays, the ozone layer both warms the air effectually information technology and protects us on the surface from the harmful short-wavelength radiation that tin crusade skin cancer.
It is of import to recognize the difference betwixt the ozone layer in the stratosphere and ozone nowadays in trace amounts in the troposphere. Stratospheric ozone is produced when energy from the sunday breaks apart Oii gas molecules into O atoms; these O atoms and so bail with other O2 molecules to form O3, ozone. This process was offset described in 1930 by Sydney Chapman, a geophysicist who synthesized many of the known facts nearly the ozone layer. Tropospheric ozone, on the other hand, is a pollutant produced when emissions from fossil-fuel burning interact with sunlight.
In a higher place the stratosphere, temperature begins to driblet again in the next layer of the atmosphere called the mesosphere, as seen in the previous figure. This temperature decrease results from the rapidly decreasing density of the air at this altitude. Finally, at the outer reaches of Globe's atmosphere, the intense, unfiltered radiation from the sun causes molecules like O2 and Northwardtwo to break apart into ions. The release of free energy from these reactions actually causes the temperature to ascent again in the thermosphere, the outermost layer. The thermosphere extends to about 500 km above Earth'south surface, still a few hundred kilometers below the altitude of most orbiting satellites.
Comprehension Checkpoint
All conditions, including clouds, current of air, and precipitation, occurs in the
Pressure in the temper
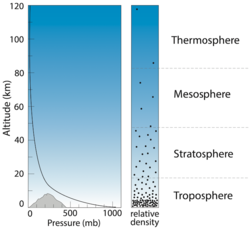
Atmospheric pressure level can be imagined as the weight of the overlying column of air. Unlike temperature, pressure decreases exponentially with altitude. Traces of the atmosphere can be detected as far as 500 km above Earth's surface, but 80 percent of the atmosphere's mass is contained inside the 18 km closest to the surface. Atmospheric pressure is generally measured in millibars (mb); this unit of measurement is equivalent to 1 gram per centimeter squared (1 chiliad/cmii). Other units are occasionally used, such as confined, atmospheres, or millimeters of mercury. The correspondence between these units is shown in the table below.
| confined | millibars | atmospheres | millimeters of mercury | |||
|---|---|---|---|---|---|---|
| 1.013 bar | = | 1013 mb | = | i atm | = | 760 mm Hg |
Table 3: Correspondence of atmospheric measurement units.
At sea level, pressure ranges from nearly 960 to 1,050 mb, with an average of 1,013 mb. At the elevation of Mt. Everest, pressure is as low every bit 300 mb. Because gas pressure is related to density, this low pressure means that there are approximately one-tertiary as many gas molecules inhaled per breath on top of Mt. Everest as at bounding main level – which is why climbers experience ever more astringent shortness of breath the higher they get, as less oxygen is inhaled with every breath.
Though other planets host atmospheres, the presence of free oxygen and water vapor makes our atmosphere unique as far as nosotros know. These components both encouraged and protected life on World equally it adult, non only past providing oxygen for respiration, but by shielding organisms from harmful UV rays and past incinerating small meteors earlier they striking the surface. Additionally, the limerick and construction of this unique resource are of import keys to understanding apportionment in the temper, biogeochemical cycling of nutrients, brusk-term local weather patterns, and long-term global climate changes.
Summary
Globe'south atmosphere contains many components that can be measured in different means. This module describes these unlike components and shows how temperature and pressure change with altitude. The scientific developments that led to an agreement of these concepts are discussed.
Central Concepts
-
World's atmosphere is made up of a combination of gases. The major components of nitrogen, oxygen, and argon remain constant over time and infinite, while trace components similar CO2 and water vapor vary considerably over both space and fourth dimension.
-
The atmosphere is divided into the thermosphere, mesosphere, stratosphere, and troposphere, and the boundaries between these layers are defined by changes in temperature gradients.
-
Pressure decreases exponentially with altitude in the atmosphere.
-
Our cognition about the temper has developed based on data from a variety of sources, including direct measurements from balloons and aircraft every bit well equally remote measurements from satellites.
What Is The Makeup Of The Atmosphere,
Source: https://www.visionlearning.com/en/library/Earth-Science/6/Composition-of-Earths-Atmosphere/107
Posted by: damianoupinedegs.blogspot.com


0 Response to "What Is The Makeup Of The Atmosphere"
Post a Comment